6-Month CRMS Demo Program
Experience the future of cleanroom monitoring with the revolutionary Cleanroom Monitoring System (CRMS), a collaboration between Kanomax USA and Elemental Machines. This cutting-edge solution offers unparalleled real-time insights into your cleanroom environment, ensuring compliance, efficiency, and safety.
With features like continuous 24/7 monitoring, customizable alerts, and seamless integration with existing systems, CRMS empowers you to maintain the highest standards of cleanliness and operational excellence. Discover how our partnership combines Kanomax’s expertise in air and particle measurement solutions with Elemental Machines’ innovative IoT platform to elevate your science.
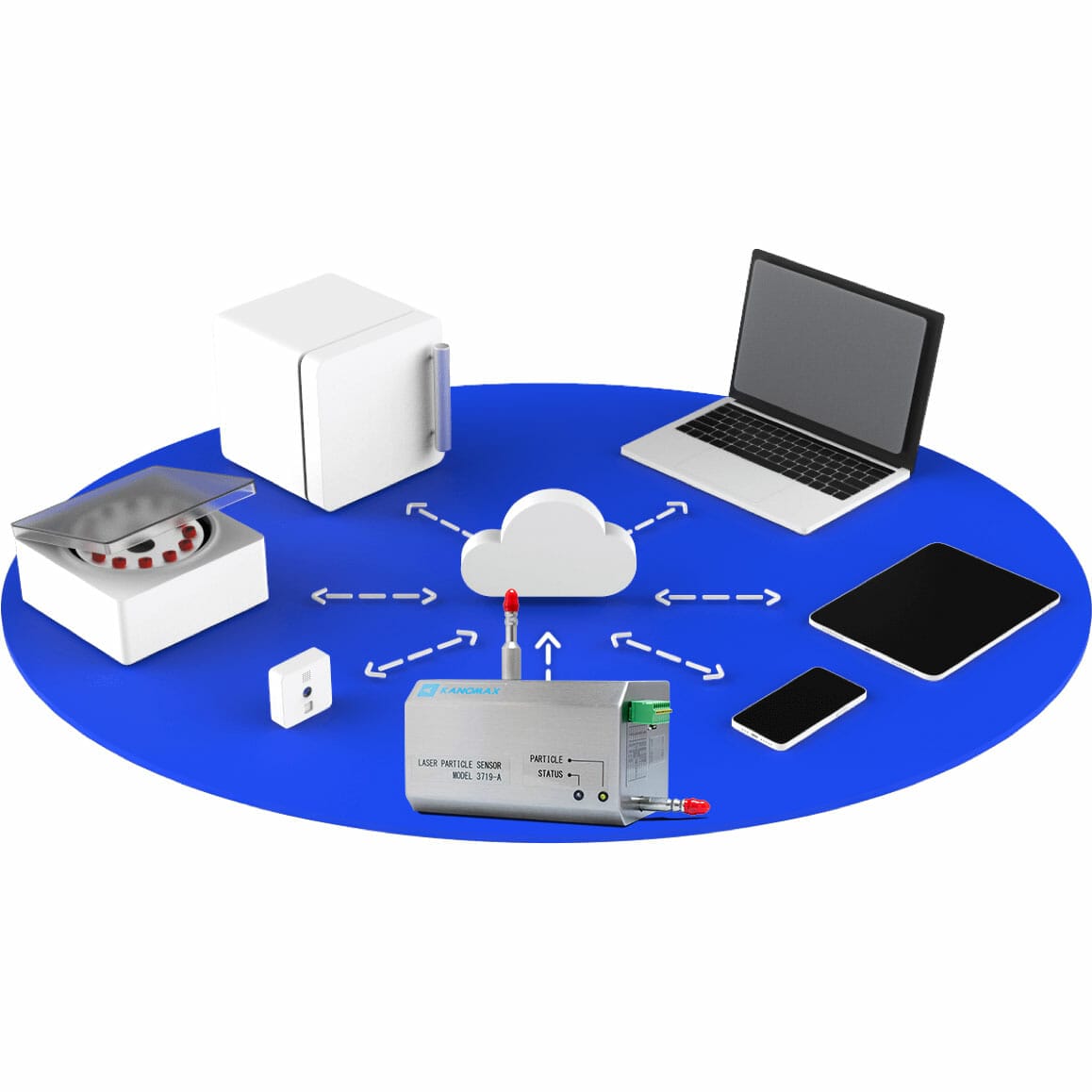
The Cleanroom Monitoring System (CRMS) provides comprehensive solutions for real-time tracking of critical environmental parameters such as temperature, humidity, particle counts, differential pressure, energy usage, and gases across multiple industries including pharmaceuticals, medical devices, aerospace, semiconductors, and automotive.
Offering both local and cloud-based data management options, the CRMS ensures seamless monitoring and prompt alarm notifications for specified events, along with reliable remote access. This system leverages Elemental Machines’ advanced technologies for enhanced integration and efficiency in maintaining optimal cleanroom conditions.
Introducing the Kanomax Cleanroom Monitoring System
Learn more about Kanomax USA’s comprehensive and customizable cleanroom monitoring systems, made in partnership with Elemental Machines.
Request a Demo Today:
Cleanroom Monitoring System (CRMS)
*Terms and Conditions Apply
More Information
Navigate the tab section below to see this product’s Features & Benefits, Specifications, Resources, and more.
- Real time monitoring of temperature, humidity, airborne particles, differential pressure, energy consumption, gases
- Cloud-based data server for monitoring data
- Initiate alarms per event
- Dependable remote access
- Security and compliant results
- System validation
- Meet all the necessary regulatory requirements: 21 CFR Part 11, ISO 14644, USP797/800
- Save money and resources for monitoring system project
- Provides a turnkey solution for a cleanroom monitoring project. Kanomax will help with:
- Developing a system proposal
- Designing monitoring system
- System installation
- System validation
- Training
- Technical support & troubleshooting
- Calibration of sensors
- Kanomax helps customers determine cleanroom monitoring requirements
- A monitoring system can be installed and begin operating within 3 months
- Cleanroom monitoring for Pharmaceutical manufacturing (FDA cGMP and EU GMP)
- Cleanroom monitoring project, which requires formal validation
- Small scale cleanroom startup project (we can offer as small as 1 sensor system)
Examples of previous project types:
- Aerospace
- Advanced Material
- Automotive parts manufacturing
- Electrics assemble
- Medical device manufacturing
- Pharmaceutical packaging
- USP797 compounding pharmacy
Data Sheet - English
Our sales representatives are here to help you find the right instrument for your application. Fast.
Request a quote today to find the product you need with expert assistance.